Trans-Arterial Micro-Embolisation (TAME):
A New Approach to Chronic Musculoskeletal Pain
Chronic joint and tendon pain — particularly knee osteoarthritis — have traditionally been managed with pain killers, injections, physiotherapy, and ultimately joint replacement surgery. Trans-arterial micro-embolisation, or TAME, offers something fundamentally different. Rather than masking pain or replacing a joint, TAME targets the underlying inflammation that drives chronic pain, treating it at its vascular source.
Developed by Dr Yuji Okuno in Japan, TAME is now performed by our interventional radiology team across multiple sites in Sydney, including Liverpool Hospital, Concord Hospital, Sutherland Hospital, Sydney South West Private Hospital, and Hurstville Private Hospital.
Why Does Chronic Musculoskeletal Pain Persist?
For decades, chronic joint and tendon pain was attributed primarily to "wear and tear." While degeneration is certainly part of the picture, it does not explain the full story. Many patients with severe changes on X-ray experience little pain, while others with modest structural damage are in significant discomfort. Pain often fluctuates in ways that do not correlate with the degree of structural change.
Recent research has revealed that chronic inflammation plays a much larger role than previously recognised. When tissue is injured — whether through a single event or repetitive stress — the body mounts an inflammatory response to promote healing. Normally, once healing is complete, this inflammation resolves. In some cases, however, the healing process fails to switch off. This is sometimes called "failed healing." The inflammation becomes self-perpetuating, continuing and even intensifying despite no ongoing injury.
Abnormal Blood Vessels and Nerves: The Source of Pain
When chronically inflamed tissue is examined under the microscope, a consistent finding emerges — clusters of abnormal, newly formed blood vessels called neovessels. Unlike normal blood vessels, which have well-organised walls and regulate blood flow effectively, these neovessels are structurally deficient. Their walls are incomplete and leaky, contributing to ongoing swelling and delivering a continuous supply of inflammatory cells and chemicals to the affected area.
New nerve fibres grow alongside these abnormal blood vessels. These nerves serve no useful purpose. They do not provide normal sensation or protective pain signals. Instead, they are densely packed with pain receptors, fire at lower thresholds than normal nerves, and can even generate pain signals spontaneously. This is why chronic musculoskeletal pain often seems out of proportion to the degree of structural damage visible on imaging.
The combination of abnormal blood vessels feeding inflammation and abnormal nerves generating pain creates the engine that drives chronic musculoskeletal conditions.
How TAME Works
TAME works by cutting off the blood supply to these abnormal vessels. Using a fine catheter inserted through a small artery — typically at the foot or wrist — an interventional radiologist navigates to the arteries supplying the affected joint or tendon. Contrast dye is injected and X-ray images are taken to identify areas of abnormal blood vessel proliferation, which appear as a characteristic "blush" — a cloud-like staining pattern distinctly different from normal vascular anatomy.
Only arteries showing this abnormal blush are treated. A small amount of embolic material is injected to block the pathological vessels. Normal arteries supplying healthy tissue are left untouched.
When the abnormal blood vessels are blocked, two things happen. First, the delivery of inflammatory cells and chemicals to the affected area stops, allowing the chronic inflammation to resolve. Second, the abnormal pain-signalling nerves that grew alongside the abnormal vessels regress and degenerate. The result is not just reduced inflammation but a reduction in the pain-generating nerve network itself.
Beyond symptom relief, there is evidence that TAME may have disease-modifying effects. In knee osteoarthritis, chronic inflammation accelerates cartilage breakdown. By reducing inflammation, TAME may slow disease progression, potentially delaying or even preventing the need for joint replacement.
The Embolic Agent
The most commonly used embolic material for TAME is imipenem-cilastatin. Originally developed as an antibiotic, it was discovered that when mixed with contrast dye, this agent forms tiny water-soluble crystals with ideal properties for this application.
These crystals are small enough to reach the abnormal microvessels but large enough to temporarily block them. The blockage lasts approximately 30 to 120 minutes before the crystals dissolve. This temporary nature is an important safety feature — if any normal vessels are inadvertently affected, the blockage resolves on its own and normal blood flow is restored.
What makes this agent particularly effective is its differential effect. When the temporary blockage clears, normal healthy arteries reopen and resume their function. However, the abnormal neovessels — with their structurally deficient walls — cannot recover from even this brief interruption. They close permanently. The result is selective, permanent elimination of the pathological vessels using a temporary agent, with minimal risk to normal tissue.
Alternative permanent embolic agents exist and produce comparable pain relief, but they carry a slightly higher risk profile because any unintended blockage of normal vessels would be permanent rather than self-resolving. For this reason, imipenem-cilastatin is generally preferred. Novel purpose-designed temporary embolic agents are under development and will soon become available in Australia.
Knee Osteoarthritis
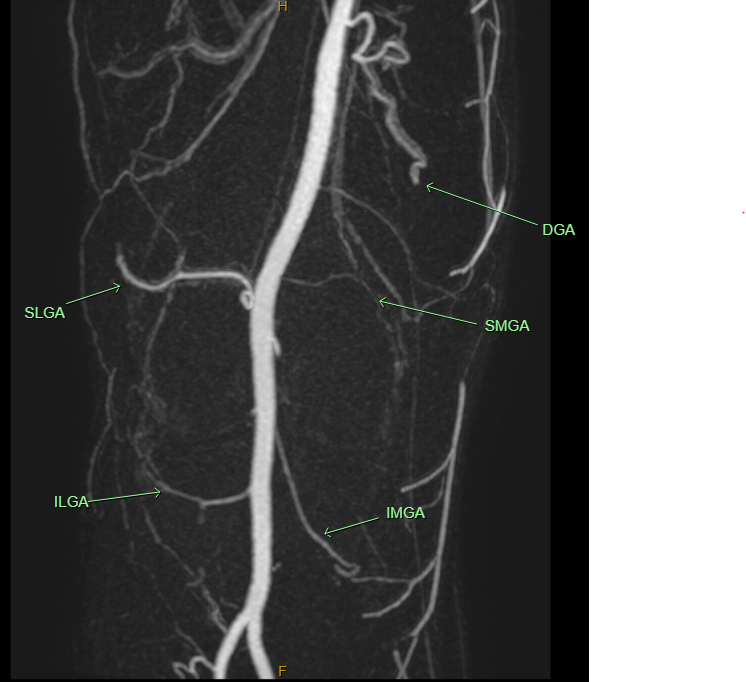
Knee osteoarthritis is the most established and extensively studied application for TAME. The knee joint is supplied by up to six genicular arteries forming a network around the joint. During the procedure, each of these arteries is examined with contrast injection. Only those showing abnormal blush — indicating active inflammation — are treated.
TAME works best for patients with moderate osteoarthritis, corresponding to Kellgren-Lawrence grades 2 and 3 on X-ray. At this stage, inflammatory processes are active and responsive to treatment while structural damage has not yet progressed to end-stage disease. Success rates in these patients are highest, with many experiencing significant and lasting pain reduction and improved function.
Patients with more advanced disease (KL grade 4) may still benefit, though the success rate is lower. At this stage, mechanical factors such as bone-on-bone contact play a larger role in generating pain, and inflammation — while still present — is no longer the dominant driver. Even so, addressing the inflammatory component can provide meaningful improvement for some patients.
Early-stage osteoarthritis (KL grade 1) does not typically warrant TAME, as conservative management remains appropriate.
TAME fills a critical gap in the treatment of knee osteoarthritis. Previously, patients who had exhausted conservative measures but were not ready for or suitable for knee replacement had limited options. TAME now serves as an intermediate therapy that may prolong time until knee replacement, provide long-term symptom control in patients who cannot undergo surgery, or in some cases avoid the need for surgery entirely.
TAME can also be combined with other treatments in the same session, including corticosteroid injection into the joint, genicular nerve ablation to address the neural pain pathways, or platelet-rich plasma injection. Whether to combine treatments or stage them separately depends on your individual circumstances and is discussed during consultation.
Clinical studies have consistently shown significant pain reduction — typically 50 to 70 percent improvement — along with improved function, reduced need for pain medications, and durable benefit extending beyond 12 months in many patients.
MR angiography showing the genicular arteries of the knee.
Post-Knee Replacement Pain
A subset of patients continue to experience pain after technically successful knee replacement surgery. This post-replacement pain often includes an inflammatory component related to surgical trauma and the presence of prosthetic material. TAME has shown utility in this challenging situation, targeting the inflammatory neovascularisation that can develop around the prosthesis or in surrounding tissues.
Knee Haemarthrosis
Haemarthrosis — bleeding into the knee joint — is another indication where TAME shows excellent results. This can occur spontaneously, in patients with inherited bleeding disorders such as haemophilia, after knee surgery, or in patients on blood-thinning medications. Chronic or recurrent haemarthrosis causes joint damage through iron deposition and inflammation. TAME addresses the vascular source of bleeding, embolising the abnormal or fragile vessels responsible for recurrent haemorrhage.
Frozen Shoulder
Adhesive capsulitis — commonly known as frozen shoulder — involves inflammation of the shoulder capsule, causing progressive pain and severe restriction of movement. The condition typically follows a prolonged course lasting 12 to 36 months, with some patients left with permanent residual restriction. Conventional treatments including physiotherapy, anti-inflammatory medications, steroid injections, and manipulation under anaesthesia offer limited effectiveness.
TAME targets the inflammatory process at its vascular source. The inflamed shoulder capsule exhibits the same pathological neovascularisation seen in other chronic inflammatory conditions. By identifying and embolising these abnormal vessels, TAME effectively shuts down the inflammation immediately. While systemic or injectable anti-inflammatory drugs act on inflammatory chemicals after they are released, TAME prevents their delivery altogether by stopping blood flow to the inflamed tissue.
Results in frozen shoulder can be striking. Many patients notice reduced pain within 24 hours of the procedure, with progressive recovery of range of motion over the following weeks. This speed of response represents a dramatic contrast to conventional management.
TAME for frozen shoulder can be performed via wrist or groin access.
Plantar Fasciitis
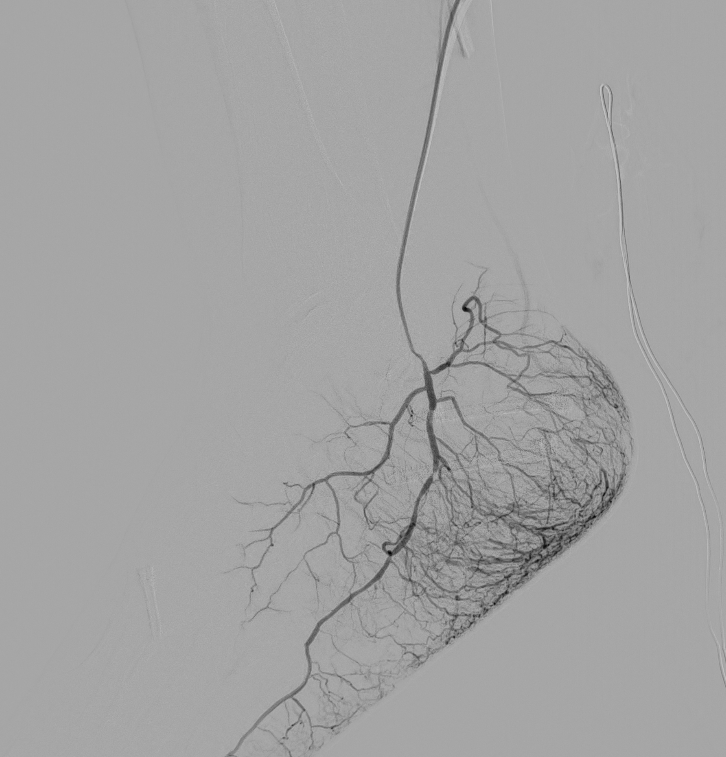
Plantar fasciitis — inflammation of the thick band of tissue running along the sole of the foot — is one of the most common causes of heel pain. The conventional radiological approach is corticosteroid injection along the plantar fascia. While this can provide relief, the injection itself is notoriously painful, effects are often short-lived, and there is approximately a 3 percent risk of plantar fascia rupture. Corticosteroids can also cause atrophy of the protective fat pad at the heel.
TAME offers a fundamentally different approach. Rather than injecting medication into an already inflamed and painful structure, TAME targets the vascular supply feeding the inflammation. It is not associated with increased rupture risk, is generally better tolerated than direct fascia injection, and addresses the underlying pathology rather than providing purely symptomatic relief.
Angiography of the foot showing marked hypervascularity (blush) of the plantar fascia.
Other Applications
Beyond the knee, shoulder, and plantar fascia, TAME is being applied and investigated for a growing range of musculoskeletal conditions. These include lateral epicondylitis (tennis elbow), medial epicondylitis (golfer's elbow), hip pathology including greater trochanteric pain syndrome and hip osteoarthritis, and small joint arthritis of the hand and foot including thumb base arthritis.
Facet joint arthropathy — a common cause of back pain — is currently being evaluated in clinical trial settings. Early data are encouraging but this remains investigational. Patients with facet-mediated back pain may be eligible for enrolment in clinical trials.
What to Expect
TAME is performed as a day case. The procedure typically takes approximately one hour, with a total time of about half a day from arrival to discharge.
Most procedures are performed under local anaesthesia alone. The access site is numbed and the procedure itself is generally well tolerated. Conscious sedation is available for patients who are anxious, and general anaesthesia can be arranged if needed or preferred.
Our team has particular expertise in transpedal access — entering through a small artery in the foot rather than the groin. This approach offers improved comfort, faster recovery, no requirement for prolonged bed rest, and the ability to proceed even if you are taking blood-thinning medications. Wrist or groin access is used when more appropriate for the specific procedure.
After the procedure, a small pressure dressing is applied to the puncture site for one to two hours. Once removed and haemostasis is confirmed, you can get up and go home.
In the days following the procedure, some soreness and bruising at the puncture site is normal. You may experience a temporary increase in pain at the treatment site for several days — this reflects the tissue's response to the embolisation and does not indicate a problem. It settles with simple pain relief.
The timeline for benefit varies. Some patients — particularly those with frozen shoulder — notice improvement within 24 hours. For most conditions, improvement develops gradually over weeks to months, with maximal benefit sometimes taking 3 to 6 months as the inflammatory process fully resolves and tissue healing occurs.
Small skin spots may occasionally appear near the treatment site, particularly when permanent microsphere agents are used. These are tiny areas where embolic material has reached skin vessels. They are painless, resolve on their own within days to a week, and are extremely rare when imipenem-cilastatin is used.
Safety
TAME is recognised as a safe procedure with a favourable risk profile. Serious complications are rare.
Common minor effects include bruising at the puncture site and the temporary pain increase described above. Clinically significant non-target embolisation — where embolic material inadvertently blocks normal vessels — is almost unheard of with imipenem-cilastatin due to its temporary and self-resolving nature. With permanent microsphere agents, occasional transient skin spots can occur, and serious lasting complications are rare but have been reported, which is one reason imipenem-cilastatin is generally preferred.
Patients with known allergies to carbapenem or beta-lactam antibiotics should inform the team, as alternative agents can be used.
As a fluoroscopically guided procedure, TAME involves a small amount of radiation exposure. Procedural times are relatively short and modern equipment uses dose-reduction technology. The radiation dose is well within accepted limits.
Is TAME Right for You?
TAME may be appropriate if you have chronic musculoskeletal pain that has not responded adequately to conservative treatments such as physiotherapy, anti-inflammatory medications, and injections, but you are not yet ready for or suitable for surgery.
TAME is not appropriate for all conditions. It is not suitable for rheumatoid arthritis or other autoimmune inflammatory conditions, which require systemic disease-modifying medications. It is not used for joint infections, which require antibiotics and sometimes surgical drainage. And it is unlikely to help when pain is driven primarily by mechanical problems such as severe joint deformity with varus or valgus angulation, extensive meniscal tears with locking or catching, or ligament instability. In these situations, orthopaedic surgical referral is more appropriate.
When TAME is not suitable, our specialists can discuss alternatives including nerve blocks, radiofrequency ablation, joint injections, or surgical referral.
The KOGAE Clinical Trial
Our team is actively conducting the KOGAE clinical trial, a research study evaluating the effectiveness of genicular artery embolisation for knee osteoarthritis. Participation offers significant benefits: the procedure and all associated imaging including MRI are provided free of charge, and you receive structured follow-up with close monitoring of your progress.
A valid GP or specialist referral is required. Contact our team to discuss eligibility and enrolment.
Our Team
The TAME programme is led by Dr Ross Copping, clinical lead in musculoskeletal embolisation and research lead for clinical trials at Liverpool Hospital and Spectrum Interventional Radiology in partnership with the Ingham Institute. He is a well recognised expert in TAME and recently presented on trans-pedal access for GAE at the inaugural VENTI conference in Tokyo 2025.
The TAME team also includes Dr Yehia El Hgar, Dr Shady Osman, Dr Chandra Annabattula and Dr Hao Xiang. All five members of the TAME team are involved in the KOGAE clinical trial.
Dr Ross Copping presenting on trans-pedal genicular artery embolisation at VENTI 2025
Dr Shady Osman, Dr Hao Xiang, Dr Ross Copping, Dr Akshay Kohli and Dr Chandra Annabattula at VENTI 2025
Pre-Procedural Assessment
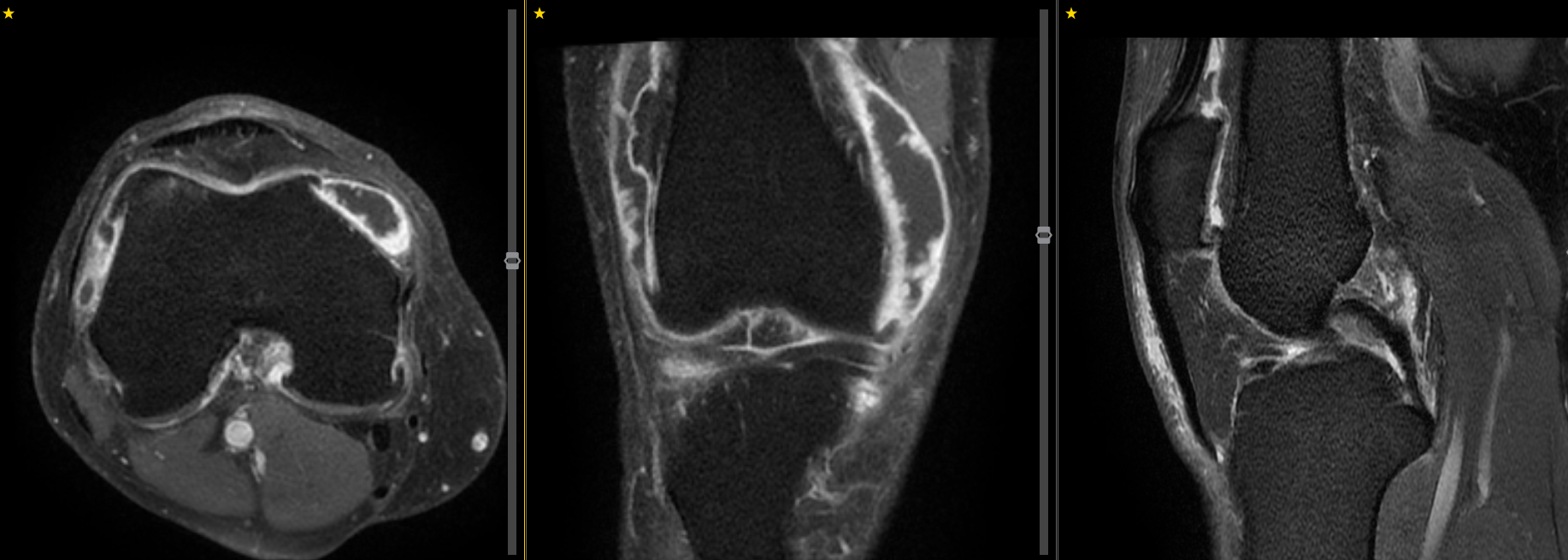
Patients considering TAME undergo a comprehensive consultation including review of your symptoms, physical examination, and assessment of all available imaging. For knee osteoarthritis, this typically includes weight-bearing X-rays, CT angiography of the knee to map the vascular anatomy, and MRI to identify areas of active inflammation.
You will also complete validated questionnaires — such as the WOMAC or KOOS scores for knee conditions — to provide a baseline measurement of your pain and function. These are repeated at follow-up to objectively track your improvement.
Follow-up appointments are scheduled at 1 month, 3 months, and 6 to 12 months after the procedure to assess your response and discuss any further treatment if needed.
MRI demonstrate marked synovial hyperenhancement in a patient with knee osteoarthritis. A great target for TAME.
How to Access Treatment
A referral from your GP or specialist is required. Please bring all imaging studies, any recent blood test results, and a complete list of your current medications — particularly blood thinners, anti-inflammatory drugs, and any immunosuppressive medications.
Treatment is available through the public hospital system at Liverpool, Concord, and Sutherland Hospitals. Privately insured patients can be seen at Sydney South West Private Hospital or Hurstville Private Hospital.
Patients who join the KOGAE clinical trial can receive the procedure and all associated imaging free of charge.